Ad VitaCart offers vitamins health supplements organic health products. Software for administrative support of a health care facility.
 21st Century Cures And You A Guide To Understanding The 21st Century Cures Act Science In The News
21st Century Cures And You A Guide To Understanding The 21st Century Cures Act Science In The News
Shop from our selection of over 45000 products with discount.
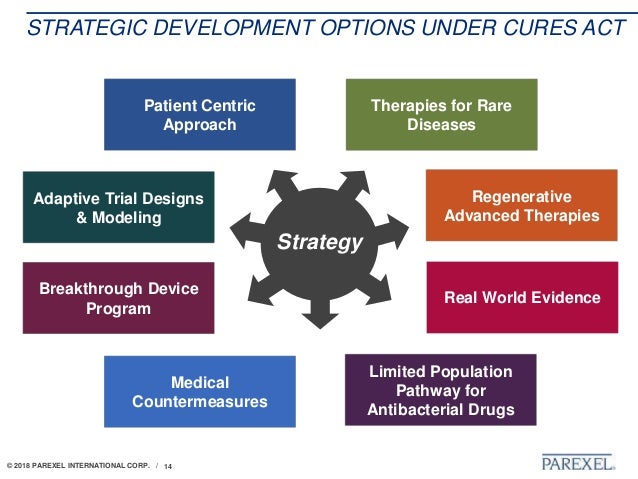
The 21st century cures act. Interoperability Information Blocking and the ONC Health IT Certification Program. Designed largely to accelerate medical product development and get new medications and treatments through the development process faster the 21st Century. In March 2020 unrelated to but somewhat obscured by the coronavirus disease 2019 pandemic the Office of the National Coordinator for Health Information Technology at the US Department of Health and Human Services announced a new landmark medical informationsharing rule referred to as the 21st Century Cures Act final rule.
In December 2016 President Obama signed the 21st Century Cures Act Cures Act into law. Ill use the Cures Rule from here on since thats what were talking about. House of Representatives and Senate with strong bipartisan support and was signed into law on December 13 2016.
The part about the electronic sharing of records is in the ONC Cures Act Final Rule or the Cures Rule. It seeks to increase health data exchange and limit refusals to share health data. 21st Century Cures Act FAQs 1 Q1 What is the 21st Century Cures Act.
21 st Century Cures Act. On May 1 2020 the US. 1 The rule is complex and governs many facets of health.
Shop from our selection of over 45000 products with discount. The rule establishes interoperability requirements that promote innovation in patient data delivery and prevents information blocking. The 21st Century Cures Act The Cures Act or the 21st Century Cures Act passed overwhelmingly in both the US.
Not later than 6 months after the date of enactment of the 21st Century Cures Act the National Coordinator shall convene appropriate public and private stakeholders to develop or support a trusted exchange framework for trust policies and practices and for a common agreement for exchange between health information networks. The 21st Century Cures Act a bi-partisan law passed in 2016 includes a whole lot of stuff. Ad VitaCart offers vitamins health supplements organic health products.
Some of its provisions are also meant to empower patients with more information about their own health. Entrepreneur - Provisions in the Cures Act Final Rule represent huge advances in patient empowerment freedom convenience and unprecedented accessibility. The 21st Century Cures Act Cures Act which was enacted in 2016 see our blog post here excluded from the statutory definition of device five categories of software.
The 21st Century Cures Act would thus foster medical innovation by supporting government research funding but reduce the level of evidence needed to approve prescription drugs and medical devicesa step that would actually hurt innovation. Designed largely to accelerate medical product development and get new medications and treatments through the development process faster the 21st Century Cures Act is a significant piece of legislation that Congress passed in 2016. Department of Health and Human Services published the final rule.
A word on the lingo. Designed largely to accelerate medical product development and get new medications and treatments through the development process faster the 21st Century Cures Act. A1 On May 1 2020 the Office of the National Coordinator for Health IT ONC published the final rule in the Federal Register.
21st Century Cures Act The 21st Century Cures Act Cures Act signed into law on December 13 2016 is designed to help accelerate medical product development and bring new innovations and. The act has several elements of interest to health care providers including regulations designed to facilitate sharing of data for research purposes thereby accelerating drug and device development. In March 2020 the Office of the National Coordinator ONC for Health Information Technology released the interoperability and information-blocking rule as a part of the 21st Century Cures Act Cures Act.










